Transgenic Animals Book Pdf

Transgenic animals can be used as disease models transpharmers xenotransplanters food sources and other biological models.
Transgenic animals book pdf. In the interest of educating the public about transgenic animals and determining their effect on society the methods of creating transgenic animals is discussed. Read Online or Download Transgenic Animals Why ebook in PDF Epub Tuebl and Mobi. These books cost money to buy but you can get them free for review.
Caldovic Department of Genetics and Cell Biology University of Minnesota St Paul Minnesota USA There is a chronic need to develop transgenic fish for aquaculture and genetically engineered farm animals for various agricultural and medical purposes. Let us discuss a few of them. Fast download speed and ads free.
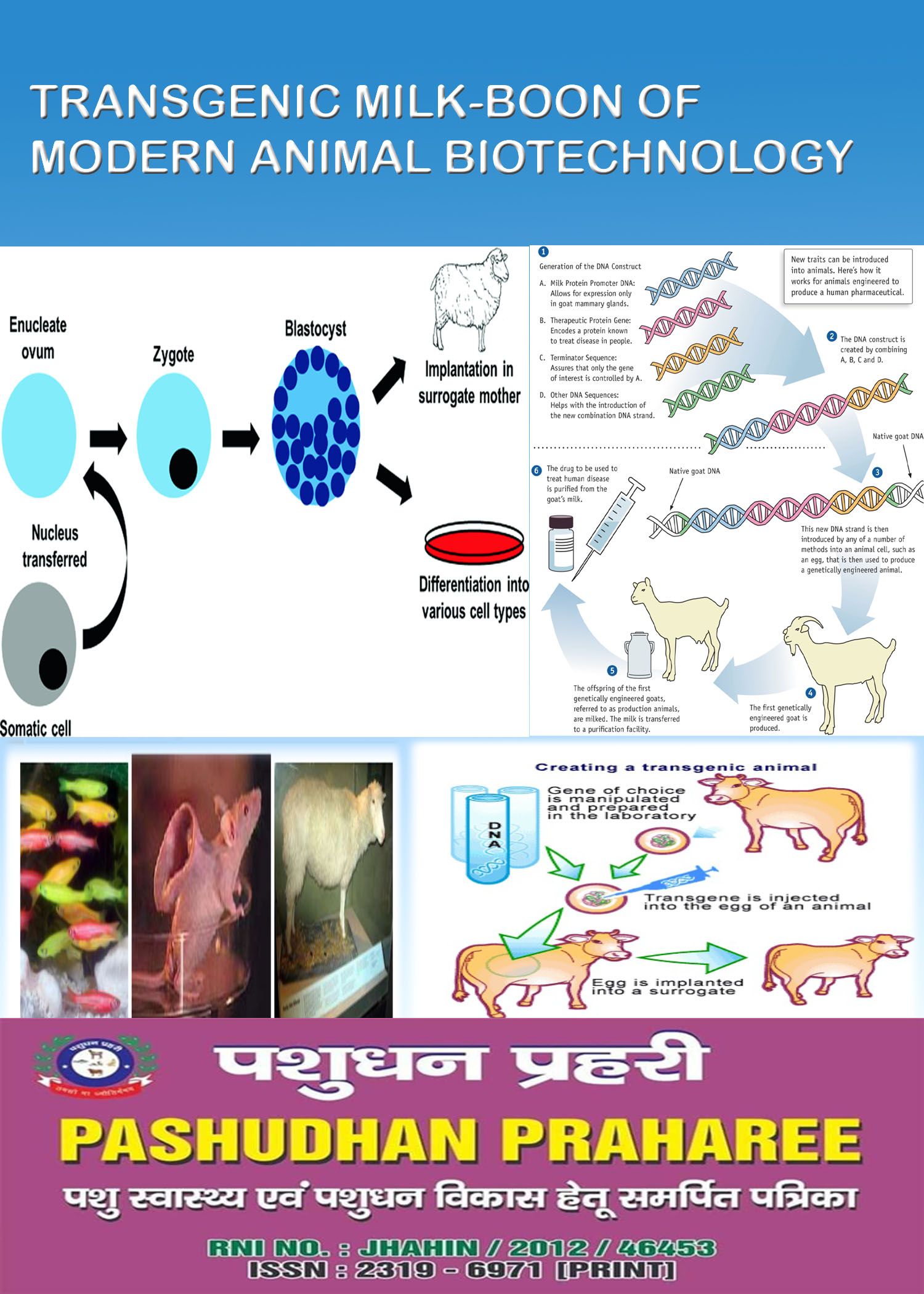
Drawn from both academia and industry the contributors to this monograph present in one concise volume all the relevant information on the different aspects of transgenesis. The foreign gene is constructed using recombinant DNA methodology. After injecting the DNA the embryo is implanted into the uterus of receptive females.
This well-illustrated handbook covers the technical aspects of gene transfer from molecular methods to whole animal considerations for important laboratory and domestic animal species. Abstract Transgenic animals are extensively used to study in vivo gene function as well as to model human diseases. Transgenic animal technologies and the ability to introduce functional genes into animals have revolutionized our ability to address complex biomedical and biological questions.
We cannot guarantee that every book is in the library. This project details transgenic animal technology applications ethics and legalities as an example of technologys impact on society. The technology for producing transgenic animals exists for a variety of vertebrate and invertebrate species.
We cannot guarantee that every books. Telos is defined as the set of Drugs from Transgenic Animals in Clinical needs and interests which are genetically based Trials environmentally expressed and which collectively The approval of ATryn rATIII by USFDA has constitute or define the form of life or way of opened gates for other drugs from transgenic living exhibited by that animal and whose animals. 29 Recombinant C1 inhibitor.